Basic Physical Concepts
Understanding fundamental physics is key to grasping electricity and electronics, and you don’t need advanced math to get started.
Atoms
All matter is made of tiny, fast-moving particles. These particles are so small and dense that matter appears continuous, even though it’s mostly empty space. Ancient civilizations guessed that matter was made of invisible particles based on their observations of different substances like water, rocks, and metals.
By the 1900s, the atomic theory was widely accepted because it explained the behavior of matter better than any other theory. Scientists identified 92 natural elements, each with unique atoms. Even a small change in an atom can significantly alter a substance’s behavior. For example, oxygen, with eight protons, supports life, while nitrogen, with seven protons, does not.
Protons, Neutrons, and the Atomic Number
An element’s identity comes from its nucleus, which contains protons and neutrons. Protons have a positive electric charge, while neutrons have no charge. Hydrogen, the simplest element, has one proton and usually no neutrons.
Helium, the second most abundant element, has two protons and two neutrons. When hydrogen turns into helium in the sun, it releases energy, making the sun shine. This process is called fusion.
The number of protons in an element’s nucleus, its atomic number, defines the element. For example, lithium has three protons, and beryllium has four.
Isotopes and Atomic Weights
The number of neutrons in an element can vary, creating different isotopes. Each element’s atomic number remains the same, but its atomic weight changes based on the number of neutrons. For instance, common carbon, C12, has an atomic weight of 12, while C14 has an atomic weight of 14.
Electrons
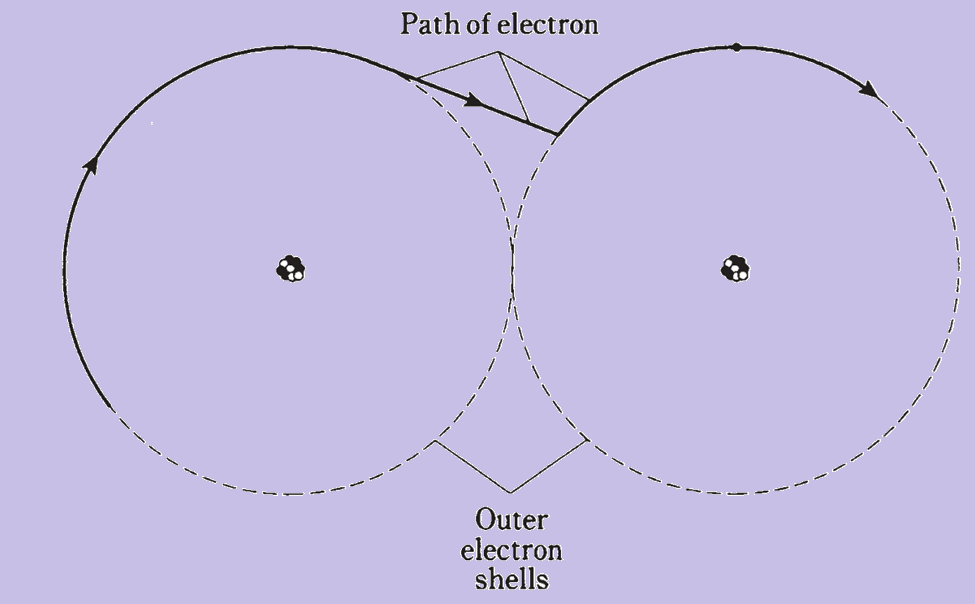
Electrons, which have a negative charge, surround the nucleus of an atom. Protons have a positive charge. Electrons and protons have equal but opposite charges. Early atomic models imagined electrons orbiting the nucleus like planets around the sun. Modern views see electrons as moving so fast that their exact location can’t be pinpointed; instead, they occupy regions called electron shells.
Electrons can move between atoms in some materials, causing electricity. They are much lighter than protons or neutrons, making their mass negligible. Generally, the number of electrons equals the number of protons, balancing the atom’s charge. However, extreme conditions can knock electrons loose, disrupting this balance.
Ions
When an atom has more or fewer electrons than protons, it becomes an ion with an electrical charge. A shortage of electrons results in a positive charge, while an excess of electrons gives a negative charge. Ionized materials, like ionized air during a lightning stroke, can conduct electricity well.
Compounds
Different elements can share electrons to form chemical compounds. For example, water is a compound formed by hydrogen and oxygen. Compounds often appear different from their constituent elements, like how water is a liquid, while hydrogen and oxygen are gases.
Molecules
Molecules form when atoms of elements join together. For instance, an oxygen molecule (O2) consists of two oxygen atoms. Water (H2O) has two hydrogen atoms and one oxygen atom. Molecules are always in motion, with their speed depending on temperature.
Conductors
Materials in which electrons move easily are called electrical conductors. Silver is the best conductor at room temperature, followed by copper and aluminum. Some liquids, like mercury, are also good conductors. Gases generally do not conduct electricity well unless they become ionized.
Insulators
Insulators prevent electrical currents from flowing. Most gases, glass, dry wood, paper, and plastics are good electrical insulators. Pure water is also a good insulator, but it can conduct some current if it contains impurities. Some metal oxides are good insulators too.
Insulators can be forced to carry current through ionization, which strips electrons from their atoms. This can occur when the material gets charred, melts, or is perforated by a spark, losing its insulating properties.
The term “dielectric” is used for insulating materials that keep electrical charges apart, preventing electron flow. Excellent insulating materials are used in electrical components like capacitors. Porcelain or glass insulators are used on high-voltage utility poles to prevent short circuits.
Resistors
Resistors conduct electricity but not as well as conductors. Their conductivity can be adjusted by adding impurities or winding a thin wire into a coil. Resistors control current flow in electronic circuits.
Electrical resistance is measured in ohms. The higher the ohms, the greater the resistance, making it harder for current to flow. In electrical systems, low resistance is desirable to minimize energy loss as heat. Thick wires and high voltages reduce resistance loss in long-distance electrical lines.
Semiconductors
Semiconductors allow electron flow but not as well as conductors. They have special properties due to the addition of impurities like indium or antimony. Semiconductors include substances like silicon, selenium, or gallium.
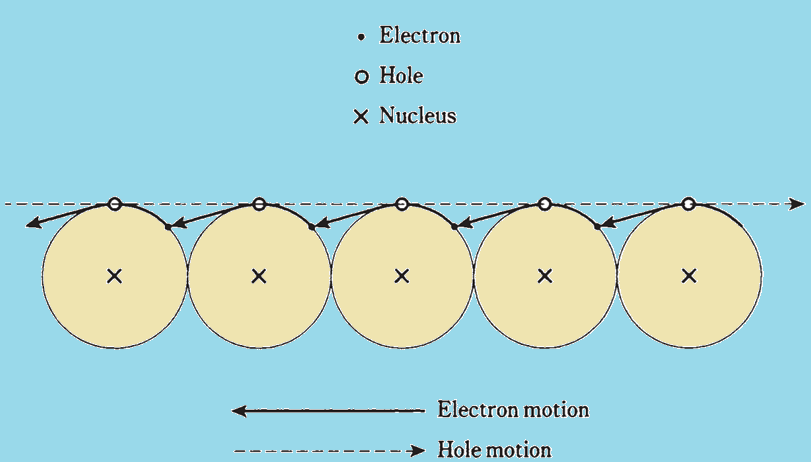
Semiconductors can be N-type (mostly electrons) or P-type (mostly holes). N-type materials have negatively charged electrons, while P-type materials have positively charged holes. Semiconductors are used in diodes, transistors, and integrated circuits.
Semiconductors make modern electronics possible, like compact computers. Without them, devices would be much larger, more expensive, and require more power.
Written by Farshid